Heart’s own immune cells can help it heal
October 29, 2014
By Julia Evangelou Strait
The heart holds its own pool of immune cells capable of helping it heal after injury, according to new research in mice at Washington University School of Medicine in St. Louis.
Most of the time when the heart is injured, these beneficial immune cells are supplanted by immune cells from the bone marrow, which are spurred to converge in the heart and cause inflammation that leads to further damage. In both cases, these immune cells are called macrophages, whether they reside in the heart or arrive from the bone marrow. Although they share a name, where they originate appears to determine whether they are helpful are harmful to an injured heart.
In a mouse model of heart failure, the researchers showed that blocking the bone marrow’s macrophages from entering the heart protects the organ’s beneficial pool of macrophages, allowing them to remain in the heart, where they promote regeneration and recovery. The findings may have implications for treating heart failure in humans.
The study is now available in The Proceedings of the National Academy of Sciences Early Edition.
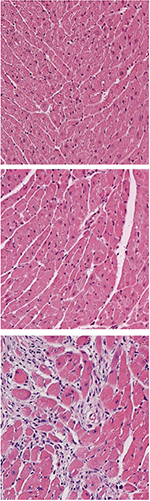
K. LAVINE
Following injury, neonatal mouse hearts (middle) heal well, appearing similar to healthy heart tissue (top). But adult hearts form scar tissue following injury (bottom).
This disparity in healing capacity was long a mystery because the same immune cells appeared responsible for both repair and damage. Until recently, it was impossible to distinguish the helpful macrophages that reside in the heart from the harmful ones that arrive from the bone marrow.
The new research and past work by the same group — led by Douglas L. Mann, MD, the Tobias and Hortense Lewin Professor of Medicine and cardiologist-in-chief at Barnes-Jewish Hospital — appear to implicate these immune cells of different origins as responsible for the difference in healing capacity seen in neonatal and adult hearts, at least in mice.
“The same macrophages that promote healing after injury in the neonatal heart also are present in the adult heart, but they seem to go away with injury,” Lavine said. “This may explain why the young heart can recover while the adult heart can’t.”
Because they are interested in human heart failure, Lavine and his colleagues developed a method to progressively damage mouse cardiac tissue in a way that mimicked heart failure. They compared the immune response to cardiac damage in neonatal and adult mouse hearts.
The investigators found that the helpful macrophages originate in the embryonic heart and harmful macrophages originate in the bone marrow and could be distinguished by whether they express a protein on their surface called CCR2. Macrophages without CCR2 originate in the heart; those with CCR2 come from the bone marrow, the research showed.
Lavine and his colleagues asked whether a compound that inhibits the CCR2 protein would block the bone marrow’s macrophages from entering the heart.
“When we did that, we found that the macrophages from the bone marrow did not come in,” Lavine said. “And the macrophages native to the heart remained. We saw reduced inflammation in these injured adult hearts, less oxidative damage and improved repair. We also saw new blood vessel growth. By blocking the CCR2 signaling, we were able to keep the resident macrophages around and promote repair.”
Some CCR2 inhibitors are being tested in phase 1 and 2 clinical trials for treating rheumatoid arthritis. But before these drugs can be evaluated in people with heart failure, more work must be done to find out whether the same mechanisms are at work in human hearts, according to the researchers.
“We have identified similar immune cell subtypes that are present in the human heart,” Lavine said. “We need to find out more about their roles in heart failure in patients and understand more about how macrophages that reside in the heart promote repair.”
This work was supported by the National Institutes of Health (NIH), the Oliver Langenberg Physician-Scientist Training Program and the Washington University Center for the Investigation of Membrane Excitability Diseases Live Cell Imaging Facility. (Grant numbers T32 HL007081, K08 HL123519, R01 HL111094, R01 HL105732.)
Lavine KJ, Epelman S, Uchida K, Weber KJ, Nichols CG, Schilling JD, Ornitz DM, Randolph GJ, Mann DL. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proceedings of the National Academy of Sciences, PNAS Early Edition. Oct. 27, 2014.
Washington University School of Medicine’s 2,100 employed and volunteer faculty physicians also are the medical staff of Barnes-Jewish and St. Louis Children’s hospitals. The School of Medicine is one of the leading medical research, teaching and patient-care institutions in the nation, currently ranked sixth in the nation by U.S. News & World Report. Through its affiliations with Barnes-Jewish and St. Louis Children’s hospitals, the School of Medicine is linked to BJC HealthCare.
No comments:
Post a Comment